Electrocatalysts
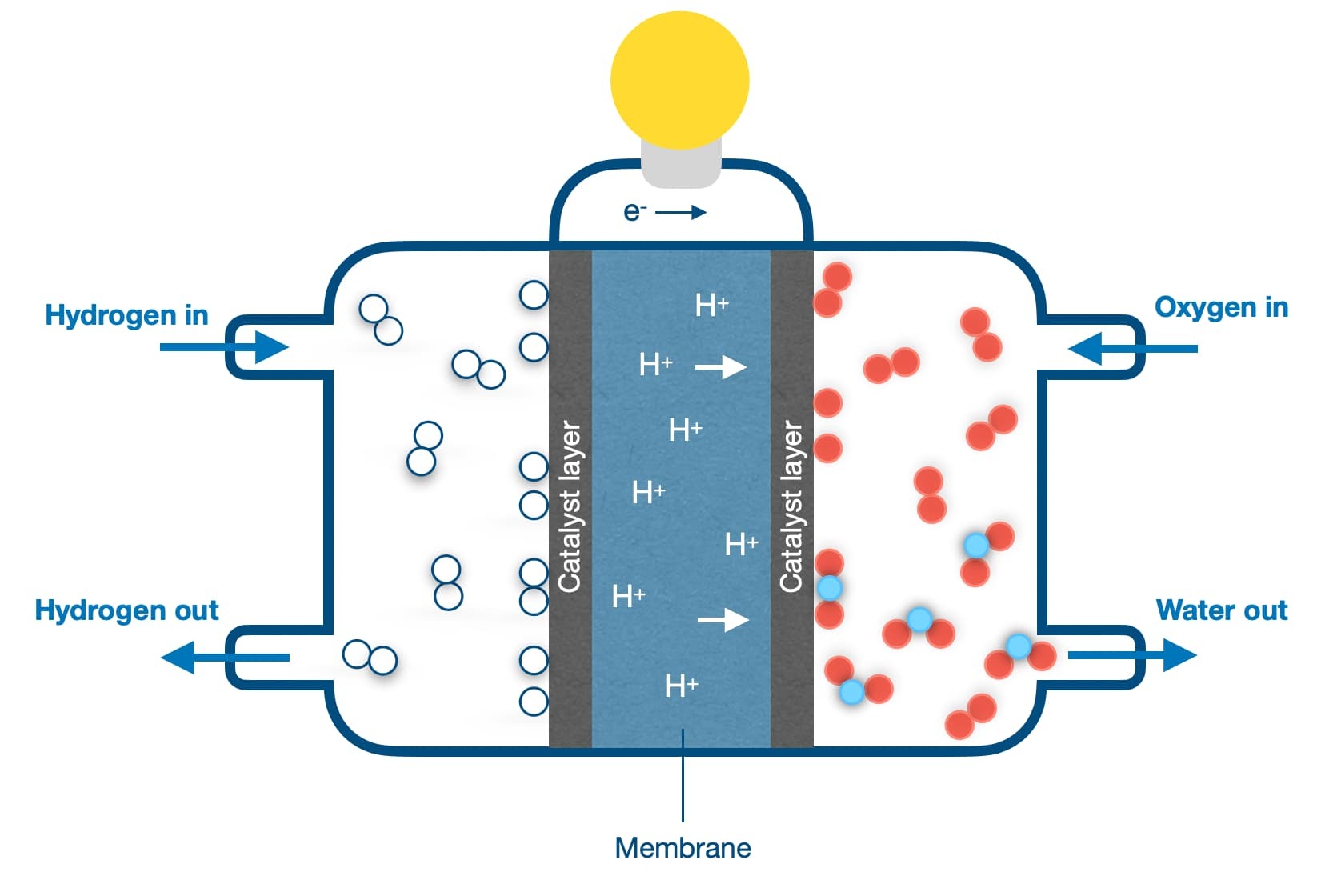
A fuel cell is a device which takes gases such as hydrogen and air as input, and produces electricity as output. This means the chemical energy which is stored in an input fuel is transformed into another form of energy, for example electricity.
A very simple fuel cell consists of two electrodes made of a metal such as platinum, immersed in an aqueous electrolyte through which only ions can flow, and not the electrons. The electrons flow through a wire connecting the two platinum electrodes, hence producing current.
More advanced fuel cells use membranes as electrolyte, which are specific types of polymers. There are several advantages in using membranes as electrolytes, size reduction and facile transportation is among them.
A key component in every fuel cell is the catalyst which governs the electrochemical reaction. The catalyst performance and stability is critical for a fuel cell to operate optimally.
C2CAT’s catalysts offer extended lifetime and durability, designed and produced with high dispersion to maximise the catalytic performance.
Check below our standard products or get in touch forcustom catalysts for your application.